Nowadays, it’s almost impossible to step into an analytical laboratory without encountering a high-performance liquid chromatography (HPLC) system, thanks to its essential applications across pharmaceuticals, food and beverages, manufacturing, and environmental monitoring.
In this article, we’ll explore what HPLC is, the principles behind its operation, and the various chromatographic techniques it employs to separate and purify complex mixtures.

High-Performance Liquid Chromatography (HPLC) is a versatile analytical technique widely used to separate, identify, and quantify components in a chemical mixture. This process relies on forcing a liquid sample, known as the mobile phase, through a column filled with a stationary phase.
As the sample passes through the column, its individual compounds or analytes interact differently with the stationary phase, causing them to separate.
Once separated, the analytes reach a detector, which records their presence. The signal from the detector is then processed by a chromatography data system (CDS) to produce meaningful results.
The final output, called a chromatogram, displays time on the x-axis and the detector’s response on the y-axis, visually representing the separated components of the mixture.
 Figure 1. Example of an HPLC chromatogram.
Figure 1. Example of an HPLC chromatogram.
How Does HPLC Work?
A typical HPLC system consists of four main hardware components: a pump, an autosampler, a column, and a detector. Supporting elements include solvents, a chromatography data system (CDS), and the tubing or capillaries that allow the continuous flow of the mobile phase and sample throughout the system.
The HPLC process generally follows these steps:
Flow of the mobile phase : The pump drives the solvent (mobile phase) through the system at a controlled flow rate.
Sample injection : The sample is introduced into the mobile phase and carried from the injection point to the top of the column.
Separation of compounds : Compounds in the sample interact differently with the stationary phase in the column, causing them to separate. The separated components then move toward the detector.
Detection of analytes : The detector identifies and measures the analytes based on specific properties that generate an electrical signal.
Chromatogram creation : The CDS translates the detector’s signal into a chromatogram, plotting analyte response against time.
Figure 2 : HPLC instrument diagram.
What Factors Affect HPLC Separations?
Several elements can impact the effectiveness of HPLC separations, including the composition of the mobile phase, the chemistry of the column, and temperature. For successful separation, analytes must interact differently with the stationary phase, making the choice of column critical for your specific compounds.
Key factors that influence the separation process include:
Properties of the analyte ,such as size, charge, polarity, and volatility.
Characteristics of the stationary phase ,including polarity, charge, and viscosity.
Nature of the mobile phase ,and how it interacts with both the analyte and the stationary phase.
Isocratic vs. Gradient Separations
HPLC separations are performed using one of two modes: isocratic or gradient.
Isocratic separation uses a constant mobile phase composition throughout the run, for example, 100% acetonitrile or a 50:50 mixture of acetonitrile and water.
Gradient separation involves gradually changing the composition of the mobile phase during the analysis. Typically, two solvents A and B are used. For instance, a run might start with 60% water (A) and 40% acetonitrile (B), with the ratio adjusted over time to improve separation.
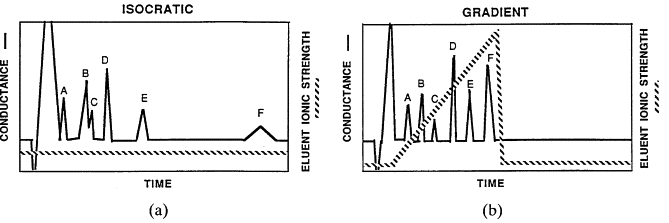
- Figure 3 : the difference between isocratic and radient Separations
Types of Liquid Chromatography Techniques
Due to the wide variety of compounds and the structural diversity of analytes, HPLC cannot be applied using a single universal method. From nanoscale to preparative-scale separations, different HPLC techniques are used depending on the specific requirements of the analysis. Below is an overview of the most commonly used HPLC methods and their typical applications.
|
|
| ||||||
High-performance liquid chromatography | HPLC operates at pressures up to 700 bar, with typical flow rates ranging from 1 to 2 mL per minute. | Comprehensive Quality Assurance and Control of Small and Large Molecules in Pharmaceuticals, Industrial Chemicals, and Food | ||||||
Ultra-high-performance liquid chromatography | UHPLC operates at pressures exceeding 1000 bar, with flow rates between 0.2 and 0.7 mL/min. The higher pressure enables improved resolution and sensitivity, faster analysis, and reduced solvent consumption compared to conventional HPLC systems. | Widely used in research and development laboratories as well as in the pharmaceutical and biopharmaceutical industries for the development and characterization of small-molecule drugs, peptides, and antibodies. | ||||||
| Liquid chromatography–mass spectrometry (LC-MS) replaces the conventional optical detectors, such as UV-Vis detectors, with a mass spectrometer for analysis. | Primarily employed for the analysis of peptides and proteins. | ||||||
| Low-flow liquid chromatography covers nano-, micro-, and capillary-flow rates, ranging from a few nL/min up to approximately 50 µL/min. By reducing the column’s inner diameter, this approach enhances sensitivity by minimizing the dilution of analyte bands. | Perfect for detecting molecules with high sensitivity in complex biological samples, where analyte concentrations may vary across several orders of magnitude. | ||||||
| Preparative LC techniques collect fractionated eluents into separate containers to isolate specific analytes, allowing purification of key components or separation of impurities for further analysis. | These techniques are used for a range of applications, from large-scale drug purification to smaller-scale processes aimed at improving product yields or isolating pure compounds. |
